COPIKTRA has an established safety profile for patients with CLL/SLL
Nonhematologic adverse reactions (ARs) occurring in ≥10% of patients receiving COPIKTRA1
Safety data are based on patients who received ≥1 prior therapy. Please see full Prescribing Information, including Boxed Warning.
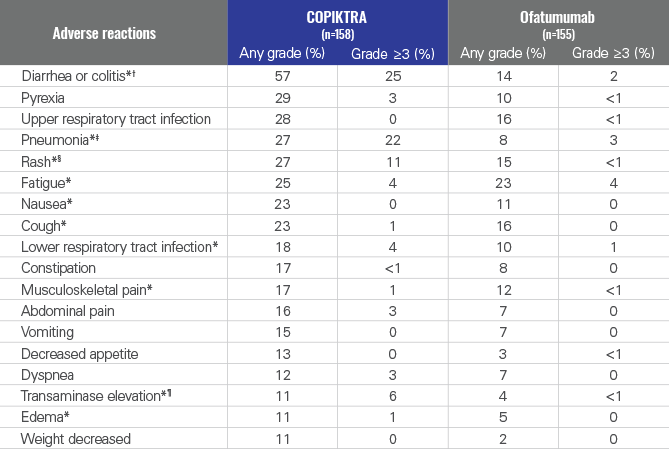
- Serious adverse reactions were reported in 73% of CLL/SLL patients (n=115/158) and most often involved infection (38% of patients; n=60/158) and diarrhea or colitis (23% of patients; n=36/158)
- COPIKTRA was dose reduced in 29% of patients due to ARs, most often due to diarrhea or colitis and rash1
New or worsening laboratory abnormalities in ≥20% of patients receiving COPIKTRA1
Safety data are based on patients who received ≥1 prior therapy.
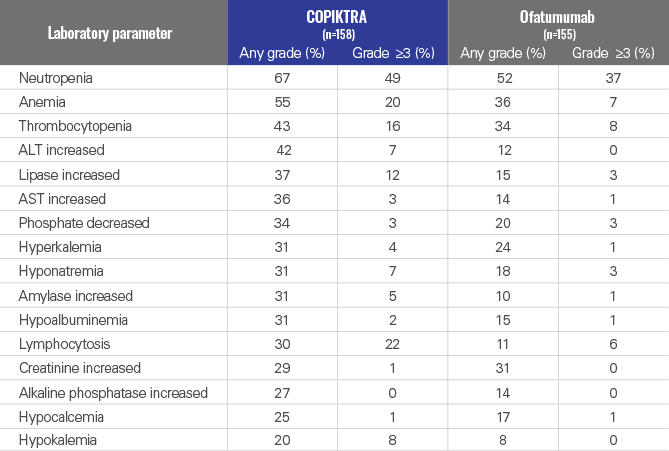
- Grade 4 laboratory abnormalities that developed in ≥2% of COPIKTRA-treated patients included neutropenia (32%), thrombocytopenia (6%), lymphopenia (3%), and hypokalemia (2%)1
- These data are not an adequate basis for comparison of rates between the study drug and the active control1
Manage adverse reactions with dose reduction, treatment hold, or discontinuation of COPIKTRA1
36% of patients with CLL/SLL (n=57/158) discontinued COPIKTRA in the DUO trial,
most often due to diarrhea or colitis, infection, and rash
- Median time to first dose modification or discontinuation was 4 months (range, 0.1 to 27 months). Of the patients requiring dose adjustments, 75% had their first dose modification or discontinuation within 7 months of starting COPIKTRA 25 mg BID||
- Median adverse event duration for diarrhea or colitis was 0.5 months (range, 1 day to 29 months), 1 month, respectively, for hepatotoxicity (range, 1 day to 16 months), pneumonitis, and cutaneous reactions (range, 1 day to 37 months)||
- *
Cutaneous reactions was defined as the onset of rash.6
- †
Hepatotoxicity was defined as an elevation in alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST).
- ‡
Median time to onset based on grade ≥3 neutropenia.6
- §
Grade ≥3 neutropenia was defined as an absolute neutrophil count <1000/mm3.
- ||
For B-cell malignancies (N=442).
- I
QR, interquartile range (25th to 75th percentile).
AR, adverse reaction.
