Patient Cases


NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend duvelisib for adult patients with or without del(17p)/TP53 mutation with relapsed or refractory CLL or SLL9*†
HIGH-RISK CLL

Joseph,‡ aged 70
Substitute teacher at nearby high school. Married with 3 grown sons. Tutors neighborhood kids in his spare time. Enjoys attending sporting events.
Status
—
ECOG PS 1 | Rai stage III
Medical history
—
CLL diagnosed 6 years ago
Summary of latest office visit
—
Patient presented with severe fatigue and pain in the upper left portion of his abdomen. Imaging showed widespread bulky lymphadenopathy with no signs suggesting Richter’s syndrome. LDH levels were slightly elevated.
Comorbidities
—
None
Cytogenetics
—
FISH: del(17p)

1st Line
Ibrutinib
- PR within 5 months
- Maintained response for 61 months before showing signs of progression
2nd Line
Venetoclax and rituximab
- After first relapse, 5-week dose ramp-up on venetoclax
- 6-month treatment regimen of venetoclax in combination with rituximab
Current
CLL appears to be refractory to treatment and imaging shows residual lymph node enlargement
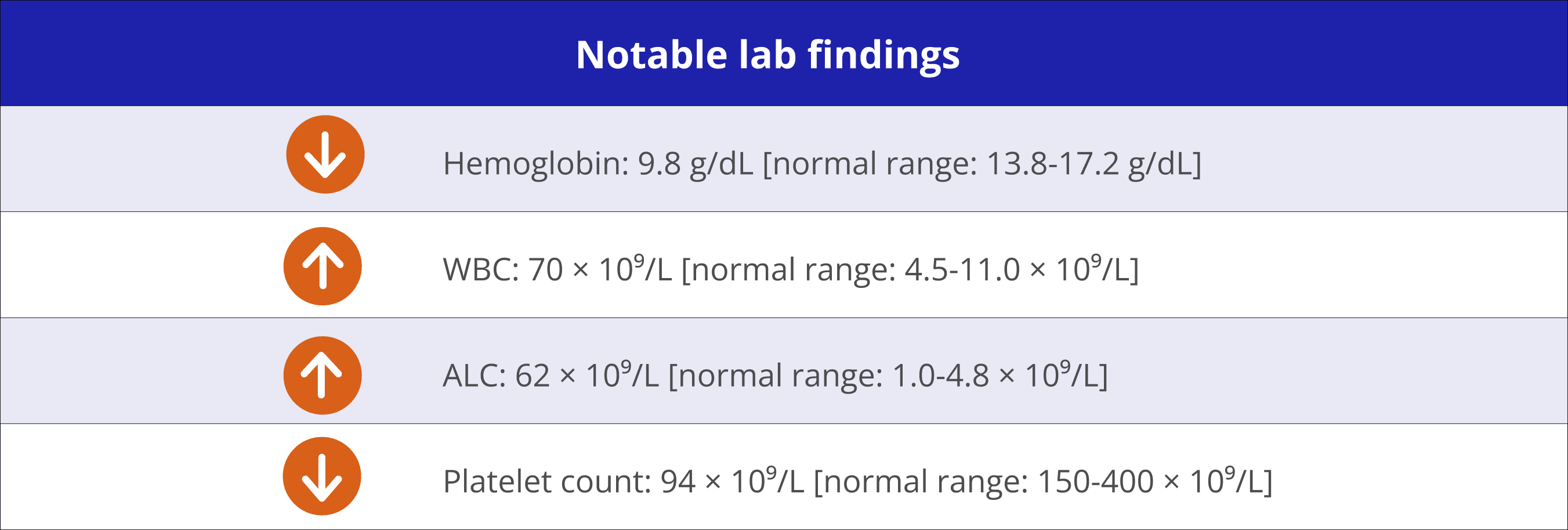
“Hearing I was high risk worried me, but I’m ready to take the next step in my treatment. I haven’t had any chemo yet, and I’d like to keep it that way. I’m happy to hear there are other options that don’t require infusions.” – Joseph‡
Consider COPIKTRA for appropriate patients with high-risk CLL.
*After prior therapy with BTK inhibitor- and venetoclax-based regimens, see NCCN Guidelines® for specific recommendation.
†NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
‡Hypothetical patient profile.
1L, first-line; 2L, second-line; 3L, third-line; ALC, absolute lymphocyte count; AR, adverse reaction; BTK, Bruton tyrosine kinase; CLL, chronic lymphocytic leukemia; ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridization; LDH, lactate dehydrogenase; NCCN, National Comprehensive Cancer Network; PR, partial response; SLL, small lymphocytic lymphoma; WBC, white blood cell count.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend duvelisib for adult patients with or without del(17p)/TP53 mutation with relapsed or refractory CLL or SLL9*†
INTOLERANT TO CURRENT THERAPY

Suzanne,‡ age 58
Ultrasound technician. Married with 2 college-aged daughters. An avid reader who looks forward to her monthly book club meetings.
Status
—
ECOG PS 1 | Rai stage IV
Medical history
—
CLL diagnosed 4.5 years ago
Summary of latest office visit
—
After several attempts to regulate and stabilize the atrial fibrillation without success, her cardiologist recommended further discussion with her oncologist to determine the best treatment option.
Comorbidities
—
Chronic kidney disease (stage IV)
Cytogenetics
—
FISH: del(11q)
Mutational analysis
TP53 wild-type
IGHV-unmutated

1st Line
FCR
- CR after 6 cycles
- 39 months in remission
before relapse
2nd Line
Acalabrutinib
- PR within 6 months
- After 3 months on acalabrutinib
maintenance, patient developed
atrial
fibrillation that could not be managed
with cardiology intervention
Current
Hematologist
discontinued treatment
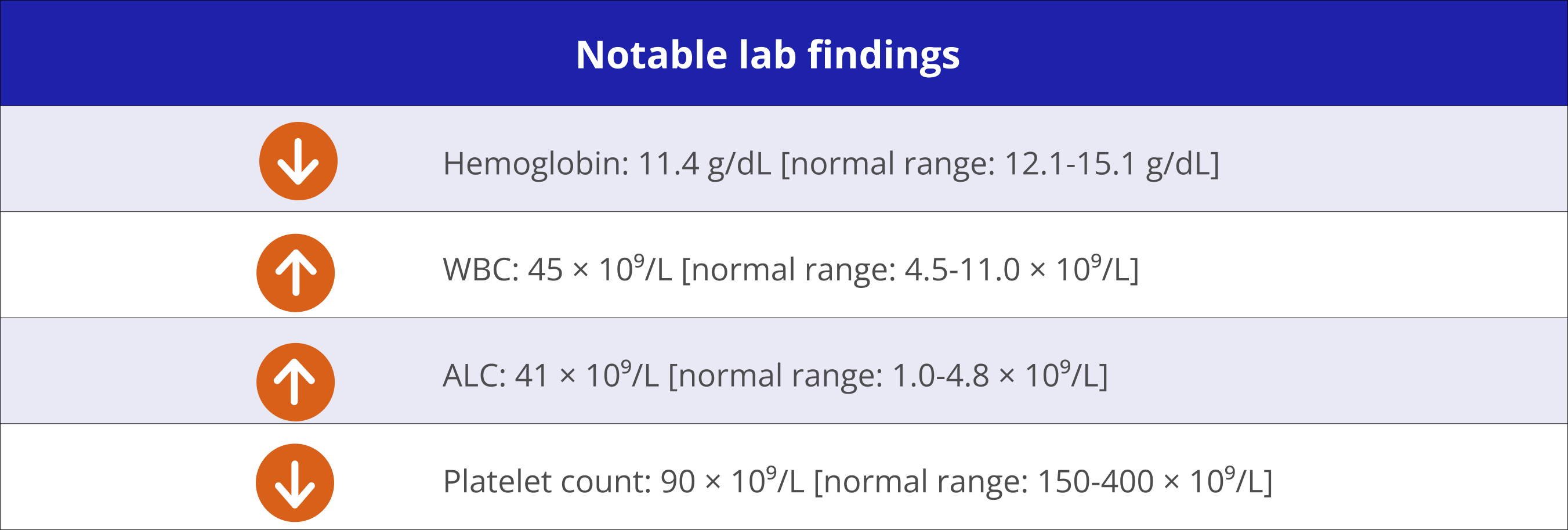
“I had a side effect with my last treatment and my doctor recommended I switch to a different medication. I’m encouraged to hear that there are other options that may be the right fit for me, and I’m ready to move forward.” – Suzanne‡
Consider COPIKTRA for appropriate patients who are intolerant to current therapy.
*After prior therapy with BTK inhibitor- and venetoclax-based regimens, see NCCN Guidelines® for specific recommendation.
†NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
‡Hypothetical patient profile.
1L, first-line; 2L, second-line; 3L, third-line; ALC, absolute lymphocyte count; AR, adverse reaction; BTK, Bruton tyrosine kinase; CLL, chronic lymphocytic leukemia; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; FCR, fludarabine/cyclophosphamide/rituximab; FISH, fluorescence in situ hybridization; IGHV, immunoglobulin heavy-chain variable region gene; NCCN, National Comprehensive Cancer Network; PR, partial response; SLL, small lymphocytic lymphoma; WBC, white blood cell count.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend duvelisib for adult patients with or without del(17p)/TP53 mutation with relapsed or refractory CLL or SLL9*†
ELDERLY AND FRAIL

Eddie,‡ aged 83
Retired accountant. Married with 2 grown sons and 4 grandchildren. Enjoys putting jigsaw puzzles together with his older grandchildren. Lives an hour away from the treatment clinic.
Status
—
ECOG PS 2 | Rai stage III
Medical history
—
CLL diagnosed 9 years ago
Summary of latest office visit
—
Physical examinations revealed left cervical lymphadenopathy and splenomegaly. Imaging showed enlarged lymph nodes.
Comorbidities
—
Osteoarthritis, hypertension
Cytogenetics
—
FISH: del(11p)
Mutational analysis
IGHV-unmutated
TP53-mutated

1st Line
Obinutuzumab and chlorambucil
- PR after 6 cycles
- Maintained response for 72 months before disease progression
2nd Line
Zanubrutinib
- PR after 6 months
Current
Shows signs of disease progression at 2-year follow-up visit
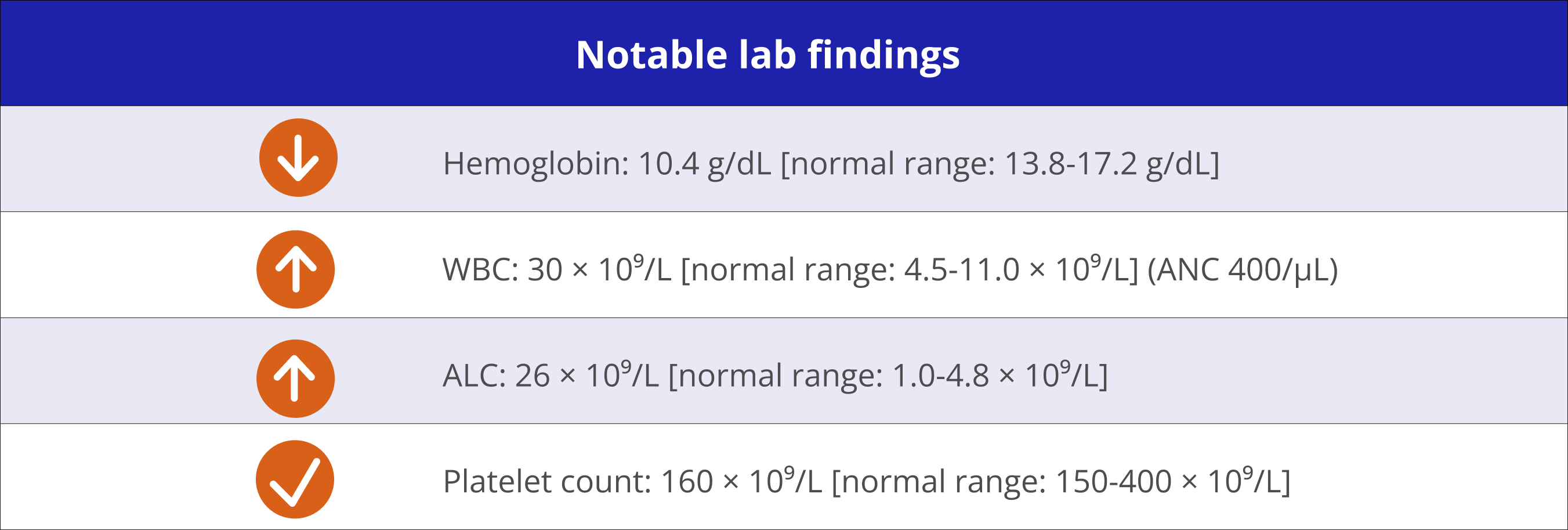
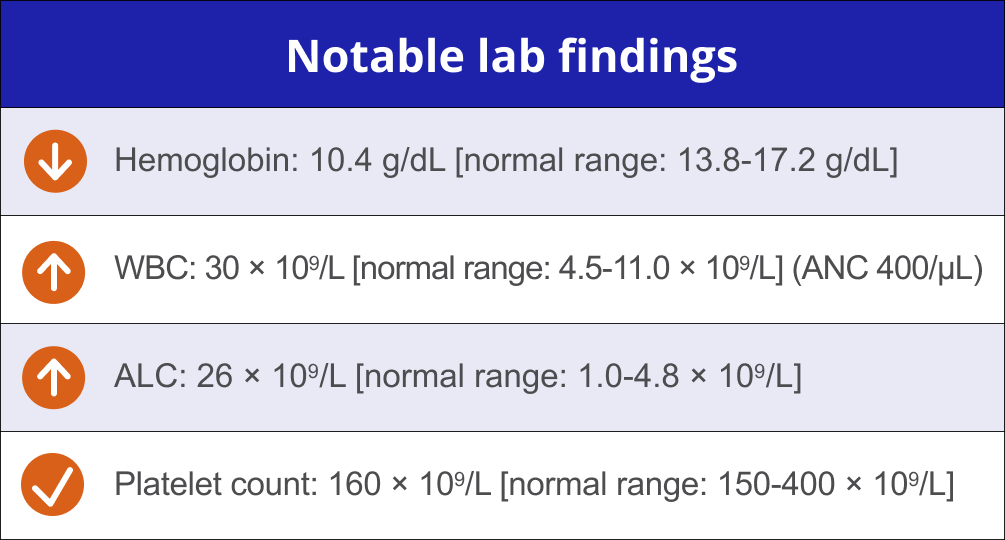
“I liked my last treatment because I didn’t have to go in for infusions and my wife got a break from driving me. I would prefer not to go back to an IV if there are oral options available.” – Eddie‡
Consider COPIKTRA for appropriate patients who are elderly and frail.
*After prior therapy with BTK inhibitor- and venetoclax-based regimens, see NCCN Guidelines® for specific recommendation.
†NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
‡Hypothetical patient profile.
1L, first-line; 2L, second-line; 3L, third-line; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; AR, adverse reaction; BTK, Bruton tyrosine kinase; CLL, chronic lymphocytic leukemia; ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridization; IGHV, immunoglobulin heavy-chain variable region gene; NCCN, National Comprehensive Cancer Network; PR, partial response; SLL, small lymphocytic lymphoma; WBC, white blood cell count.
