COPIKTRA in patients with high-risk and heavily pretreated CLL/SLL
DUO trial: a pivotal phase 3, randomized, active-controlled, multicenter, open-label superiority trial
that met its primary endpoint of progression-free survival (PFS)3
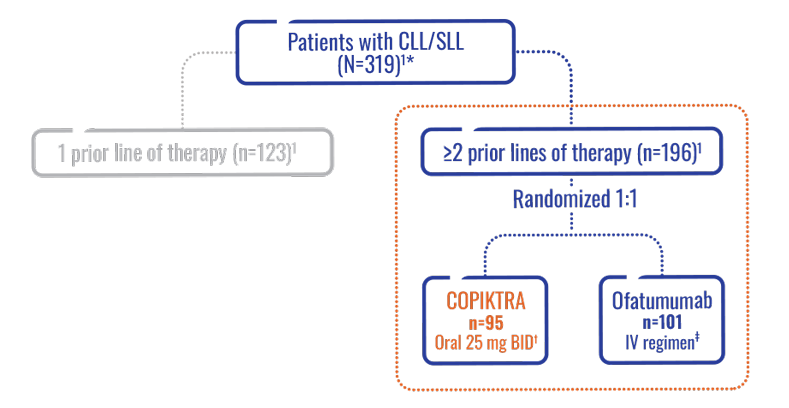
- Efficacy was based on a subset analysis of patients with at least 2 prior lines of therapy, where the risk:benefit ratio appeared greater in this more heavily pretreated population (n=196)1
- Safety was based on the comprehensive overall study population (N=319)1
Key baseline characteristics (N=319)3:
- Median age of 69 years
- ECOG performance status of 0-2
Primary endpoint1:
- PFS (IRC assessed)
Other efficacy measures endpoints3:
- Overall response rate (ORR)
- Lymph node response rate (LNRR)
- Duration of response (DOR)
Median duration of treatment on COPIKTRA was 13 months, with more than 50% of patients treated for ≥1 year§
Patients in the DUO subpopulation were heavily pretreated and relapsed/refractory (n=196)1.
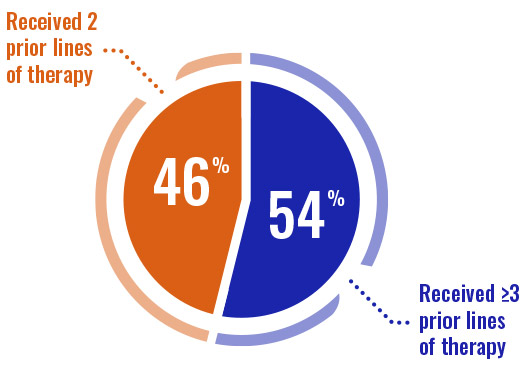
- Prior types of therapies included purine analogs (74%), alkylating agents (96%), and monoclonal antibodies (87%)3
- The median time since most recent relapse was 2.55 months before entry into DUO3
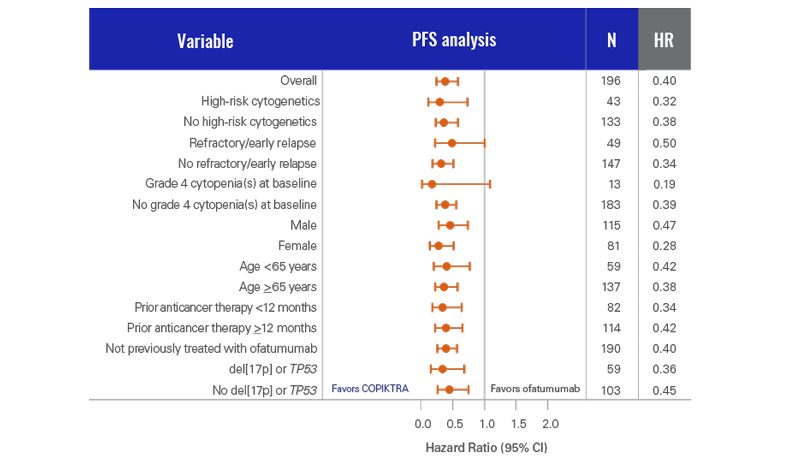
Clinically high-risk features at baseline were balanced across both treatment arms (n=196)3
Select a patient characteristic below to reveal the subpopulation percentage.
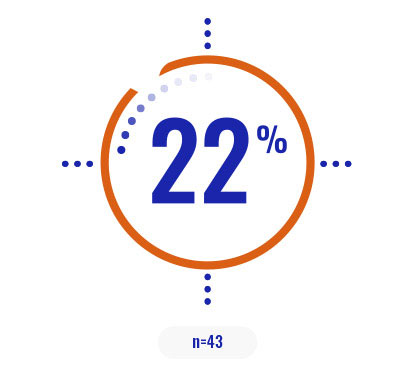
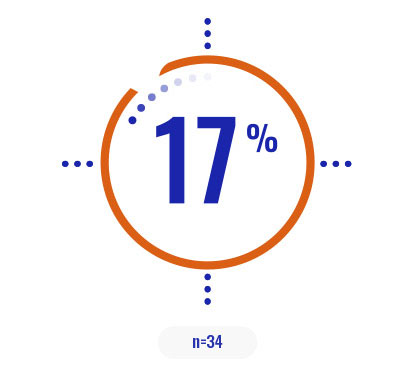
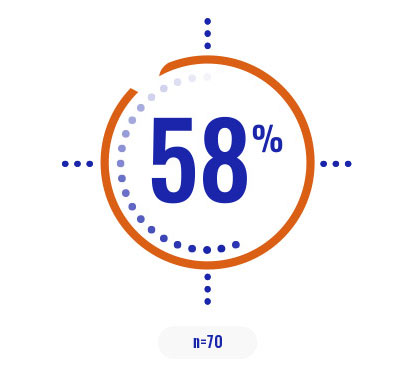
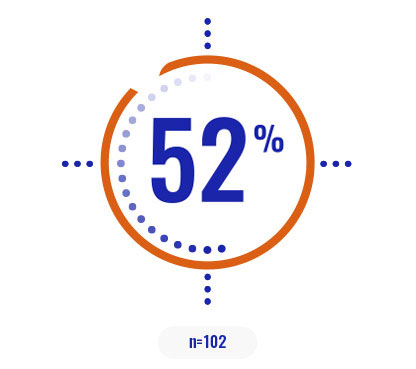
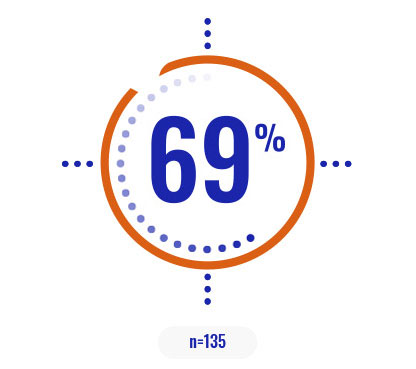
COPIKTRA may be appropriate for patients with high tumor burden or certain high-risk cytogenetics3
COPIKTRA demonstrated >7-month median PFS advantage vs ofatumumab (n=196)1*
Efficacy data are based on patients who received ≥2 prior therapies.
- COPIKTRA met its primary endpoint of PFS in the DUO subpopulation3
- There was a 62% chance of being event-free at 1 year with COPIKTRA (95% CI: 0.51-0.72) vs 34% with ofatumumab (95% CI: 0.23-0.44)3
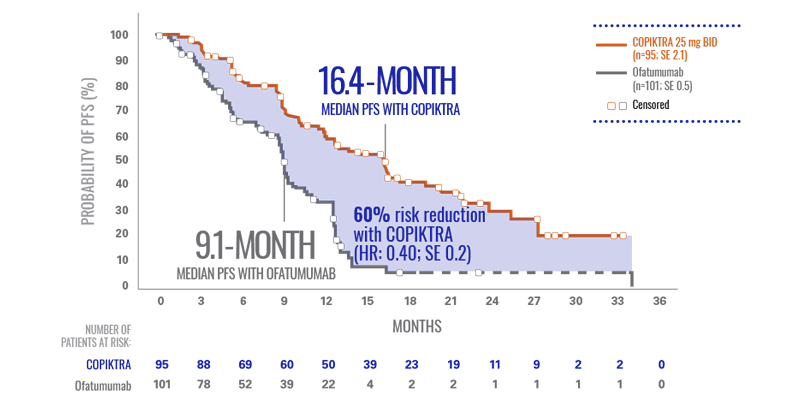
More than 60% of patients were progression-free after 1 year on COPIKTRA3
PFS analysis was conducted for COPIKTRA vs ofatumumab across patient subgroups (n=196)3
Efficacy data are based on patients who received ≥2 prior therapies.
Study features and limitations:
- This analysis was not powered to show statistical significance in PFS across these prespecified subgroups
Overall response rate: the majority of heavily pretreated patients achieved a partial response with COPIKTRA (n=196)1,3*
Efficacy data are based on patients who received ≥2 prior therapies.
- All responses were partial responses; no patient achieved a complete response1
- Data were evaluated based on the International Workshop on CLL or revised International Working Group response criteria, with modification for treatment-related lymphocytosis1
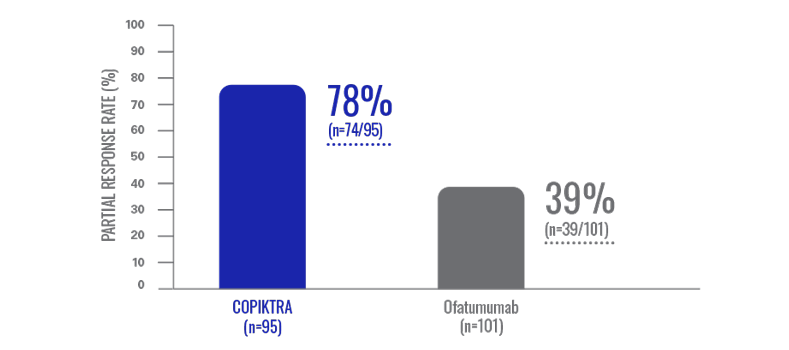
COPIKTRA provided an ORR twice that observed with ofatumumab1
Additional Data
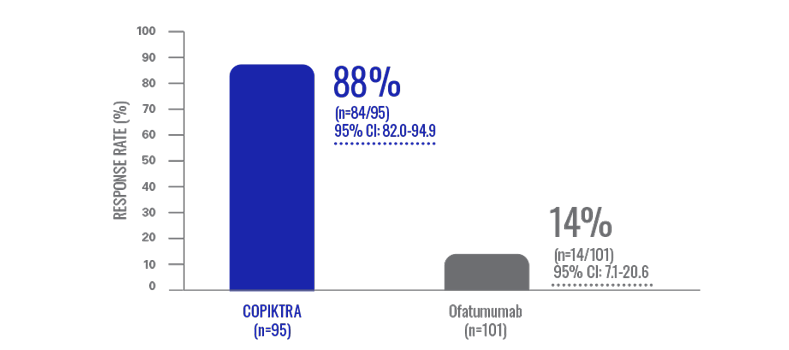
Lymph node reduction: patients experienced lymph node responses with COPIKTRA (n=196)3†
Data are based on patients who received ≥2 prior therapies.
Study features and limitations:
- LNRR was not ranked or formally tested in the hierarchy of key secondary endpoints3
- *
The International Workshop on CLL or revised International Working Group response criteria, with modification for treatment-related lymphocytosis.
- †
Lymph node response was defined as ≥50% reduction in target lesion size, as determined by the IRC.
AR, adverse reaction; CI, confidence interval; CLL, chronic lymphocytic leukemia; IRC, independent review committee; ITT, intention to treat; LNRR, lymph node response rate; ORR, overall response rate.
- *
312 patients had CLL; 7 patients had SLL (N=319).
- †
COPIKTRA was administered at 25 mg BID in 28-day treatment cycles until unacceptable toxicity or progressive disease.
- ‡
Ofatumumab was administered at an initial dose of 300 mg IV on day 1, followed a week later by 7 weekly doses of 2000 mg IV, followed 4 weeks later by 2000 mg IV every 4 weeks for 4 doses. Patients received a total of 12 doses of ofatumumab.
- §
In the subanalysis of patients with ≥2 prior lines of therapy (n=95).
- ¶
Baseline lesion ≥5 cm.
AR, adverse reaction; BID, twice daily; CLL, chronic lymphocytic leukemia; δ, delta; ECOG, Eastern Cooperative Oncology Group; γ, gamma; IGHV, immunoglobulin heavy chain variable region genes; PI3K, phosphoinositide 3-kinase; SLL, small lymphocytic lymphoma.
