Correction of Drug Information
Subject: Updated Overall Survival Data for Copiktra® (duvelisib) in Patients with Relapsed/Refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL)
01 May 2022
Dear Healthcare Professional,
Important Updated Efficacy Information in Patients with R/R CLL and SLL
This letter is to inform you about the overall survival (OS) results seen in the Final Clinical Study Report for Study IPI-145-07 (DUO), a Phase 3 study which compared duvelisib to ofatumumab in R/R CLL and SLL. Study IPI-145-07 was the basis of the approval of Copiktra® (duvelisib) by the Food and Drug Administration (FDA) on 24 September 2018.
Indication for Copiktra
Copiktra (duvelisib) is a kinase inhibitor indicated for the treatment of adult patients with R/R CLL or SLL after at least two prior therapies.
This letter is not intended as a complete description of the benefits and risks related to the use of Copiktra. Please visit the www.copiktra.com website or see enclosure for full prescribing information. Also, you may visit www.copiktrarems.com for the Fact Sheet, Medication Guide (enclosed), and other Risk Evaluation and Mitigation Strategy elements.
Background and Data Summary
Copiktra (duvelisib) is an orally administered member of the phosphatidylinositol-3-kinase inhibitor drug class, which, as a class, was the subject of an Oncologic Drugs Advisory Committee meeting on 21-April-2022. The basis for approval of Copiktra (duvelisib) in CLL/SLL was a statistically significant improvement in progression-free survival (PFS) versus the ofatumumab comparator in the subset of IPI-145-07 patients who had received two or more prior lines of therapy. Based upon an updated long term follow-up analysis report (25-June-2021), we present the following information regarding OS, which was not mature in the data submitted with the original New Drug Application.
OS in the Overall Population
In the updated data set, 90 (out of 159) patients from the ofatumumab arm crossed over to duvelisib, after disease progression. The median follow-up time for OS by the reverse Kaplan-Meier (KM) method was 63 months overall. OS was not statistically significantly different between the two treatment arms, although it appears longer for ofatumumab (hazard ratio [HR] 1.09; 95% confidence interval [CI], 0.79, 1.51; p=0.59). In the duvelisib arm the median OS was 52.3 months (95% CI, 41.8, 68.0), and in the ofatumumab arm was 63.3 months (95% CI, 41.2, NE). Although unfavorable to duvelisib, it should be noted that the CI of the HR includes the number 1, indicating there was no observed statistical difference in survival. The large confidence interval indicates a lack of precision of the estimated hazard ratio of 1.09 and the median survival estimates. With 5-years of follow-up, the mean survival times were 41.6 and 42.0 months for duvelisib and ofatumumab, respectively.
Indicated Subgroup for Patients with R/R CLL/SLL with ≥ 2 Prior Therapies
In a post-hoc updated analysis, OS did not appear to be different between the two treatment arms in the subgroup of patients with ≥ 2 prior therapies (HR 1.06; 95% CI, 0.71, 1.58). The median OS was 43.9 months (95% CI, 32.4, 56.5) in the duvelisib arm and 46.8 months (95% CI, 28.6, 74.9) in the ofatumumab arm. Again, the CI of the HR includes the number 1, indicating there was no observed statistical difference in survival. The 5-year mean survival times were 39.5 and 38.6 months for duvelisib and ofatumumab, respectively. The large CI indicates a lack of precision of the estimated HR of 1.06 and the median survival estimates. The difference was 0.9 months in favor of the duvelisib arm.
Below are the OS KM curves for the intent-to-treat (ITT) analysis set for patients who received 1 or more prior lines of therapy (overall population) (Figure 1) and for patients who received ≥ 2 prior lines of therapy (the FDA-approved label population) (Figure 2).
Figure 1: Kaplan Meier Curve for OS (ITT Population) – Duvelisib DUO Study Updated Analysis
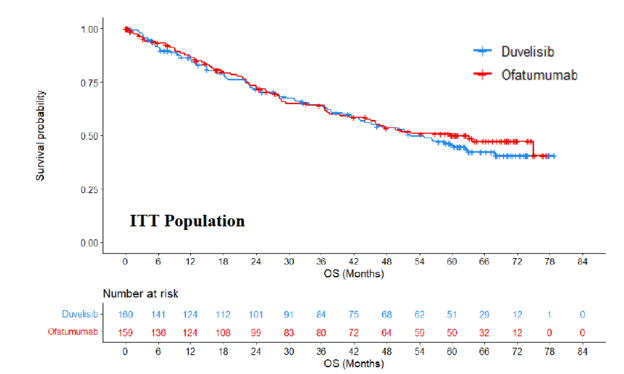
Source: FDA Analysis from PI3K Inhibitor ODAC April 21, 2022Briefing Document
Figure 2: Kaplan Meier Curve for OS (≥ 2 Prior Therapies) – Duvelisib DUO Study Updated Analysis
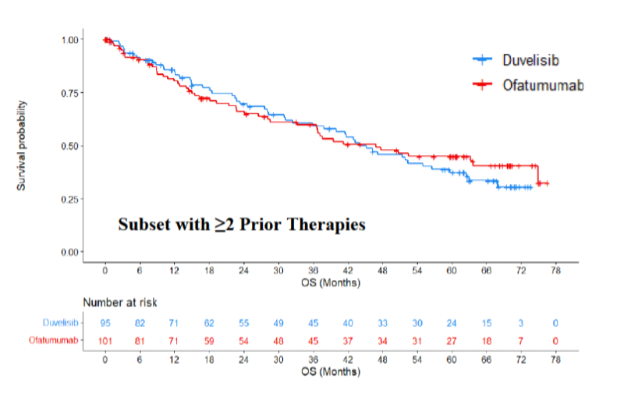
Source: FDA Analysis from PI3K Inhibitor ODAC April 21, 2022 Briefing Document
We are in active discussions with the FDA about possible revisions to the Prescribing Information and/or other relevant actions pursuant to the above information. We encourage you to integrate the above information into your decision-making processes regarding the management of relevant patients.
Reporting Adverse Events
Heath care providers and patients are encouraged to report adverse events in patients taking Copiktra (duvelisib) to Secura Bio at 1-844-9SECURA (844-973-2872) or 678-581-4536 or email securabio@parexel.com. You are also encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Sincerely,
Marci Daugherty, PharmD
Global Copiktra Scientific Lead, Secura Bio, Inc.
You may contact our medical information department at 1-844-9SECURA or visit https://securabio.com/hcp-medical-info-request, if you have any questions about the information contained in this letter and/or the safe and effective use of Copiktra (duvelisib).
Enclosure(s): COPIKTRA [package insert]. Las Vegas, NV; Secura Bio; 2021. Accessed at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/211155s005lbl.pdf, COPIKTRA [REMS Medication Guide]. Las Vegas, NV; Secura Bio; 2021. Accessed via REMS website at: https://copiktrarems.com.
© 2022 Secura Bio, Inc. All rights reserved.